Our ambition is to develop a broad portfolio of vaccine candidates in different phases, offering our whole range of expertise throughout all stages and aspects of pharmaceutical product development, e.g. preclinical and clinical studies, quality assurance and industrial manufacturing, as well as legal and commercial.
This strategy ensures that more innovations are given the opportunity to reach the market faster for the benefit of humanity. At the same time, investors are offered risk diversification with greater future leverage.
Read more how we bridge the gap between innovation and market =>
Herpes Simplex Virus type 2
– A therapeutic vaccine candidate against HSV-2
Introduction
Herpes Simplex Virus (HSV) is one of the most common infectious diseases and has followed mankind from its cradle. Yet, no efficient vaccine has been developed for the vast majority of the human population that is presumed infected by HSV, but often asymptomatic. Most people become infected as children, or in adolescence, usually through contact with a parent or other family member.

When infected, the virus travels along the sensory nerves in the skin to a nerve node where it can stay dormant for many years. From its latency, the virus can be activated during stress, sun exposure, menstruation, et cetera and migrate to the skin or mucous membrane surface where it gives rise to herpes blisters. When fought back by antivirals or the immune system, the virus goes back to its dormancy while still being infectious and transferable to new hosts.
HSV is categorised into two types; type 1 transmitted orally while type 2 sexually.
Eurocine Vaccines´ candidate is addressing the latter, HSV-2, where nearly 500 million people worldwide suffer from the infection at different stages. Presently, antivirals constitute the sole treatment to reduce symptoms, yet they do not offer a complete cure or eradication of the infection.
People frequently reach out to us, eager to see the dawn of an effective cure for their suffering, why we are highly dedicated to the quest.
Technology
The HSV-2 vaccine candidate was originally developed by the Swiss biopharma company Redbiotec AG, specialists in microbial engineering and expression of complex protein structures. In June 2022, Eurocine Vaccines, with its extensive history in vaccines, undertook the work of further development.
The development has followed a rational scientific approach consisting of a substantial number of antigenic sequences tested, where astonishing pre-clinical results could be presented – such as; reducing symptoms and obstruct the virus to reactivate while dramatically decrease shedding. This, without damaging nerve cells.
Currently two potential technologies are evaluated – Protein and mRNA. Each offering a set of unique possibilities when it comes to development and scalability in production.
A Global Concern
There is a large unmet need where public bodies recognise the medical need for both therapeutic and prophylactic HSV-2 vaccines. WHO launched a detailed report in 2019, covering the preferred product characteristics for future prophylactic and therapeutic HSV-2 vaccines. This, to urge the Life-Science Industry to concentrate its strengths in this area – estimating that the HIV acquisition rate could be reduced by 30-40% over the next 20 years, if only the genital herpes incidence is effectively reduced.
Beyond the pain and discomfort of both genital and systemic symptoms, the infection often
leads to further consequences, such as:
- Many people experience psychological burden, depression and fear of rejection, leading to mental illness that results in deteriorated quality of life and sexual relationships.
- Risk of HIV acquisition is approximately tripled in the presence of prevalent HSV-2 infection, and five times higher for those with incident HSV-2 infection.
- Neonatal herpes, that has a severe direct clinical consequence with an estimated case fatality rate of 60% without treatment. Even with treatment, neonatal herpes can lead to long- term neurological disabilities and 14 000 infants are affected annually.
- In very rare cases, the virus can also cause severe encephalitis.
WHO preferred product characteristics for herpes simplex virus vaccines =>
WHO page about Herpes Simplex Virus =>
Current Phase & Next Steps
The vaccine candidate is currently in a planning phase where the decision on which technology to pursue is the first important step. An evaluation will be made by pre-clinical studies in mice/guineapigs during 2024, to enable the decision.
In parallel, work with documentation, patents and business development is undertaken, including an early evaluation of a potential prophylactic vaccine candidate.
Following the guineapig study, we estimate to select a CDMO partner and prepare for process development and production. The time required for process development will depend on the type of active – protein or mRNA, but our preliminary ambition is to perform Toxicological studies in 2025, followed by preparation and design of the forthcoming clinical studies beginning 2026.
Product Line Extension
Prophylactic vaccine candidate
A prophylactic vaccine candidate against HSV-2 infections, according to WHO´s product characteristics and based on the existing technology, will be evaluated as a potential extension of the product line – well aligned with our business strategy to capitalise our candidates and core assets in a value adding way.
Chlamydia
– A prophylactic vaccine candidate against Chlamydia trachomatis
Introduction
Chlamydia is one of the most common sexually transmitted diseases (STD) and is caused by the bacterium Chlamydia trachomatis. It may not be considered as a major health issue but causes about 130 million worldwide infections each year. Whilst infections can be treated, they are often not detected due to patients being asymptomatic. This often result in inflammation and pelvic pain and may later lead to fatal ectopic pregnancy and/or infertility for as many as half a million women.
But not only. Chlamydia infection also increases susceptibility and transmission of other sexually transmitted diseases such as HIV.
The current treatment of chlamydia infection is with antibiotics, which most often cures the infection. However, it will not repair any permanent damage caused by the disease, which would be prevented by a vaccine. Successful implementation of prophylactic vaccination would reduce the current extensive use of antibiotics and thus reduce the evolutionary pressure towards antibiotic resistance.
Notably, non-sexually transmitted strains of Chlamydia trachomatis are the world’s leading cause of preventable blindness.
Meet the inventors and learn more about the origin of our candidate in this short video.
Technology
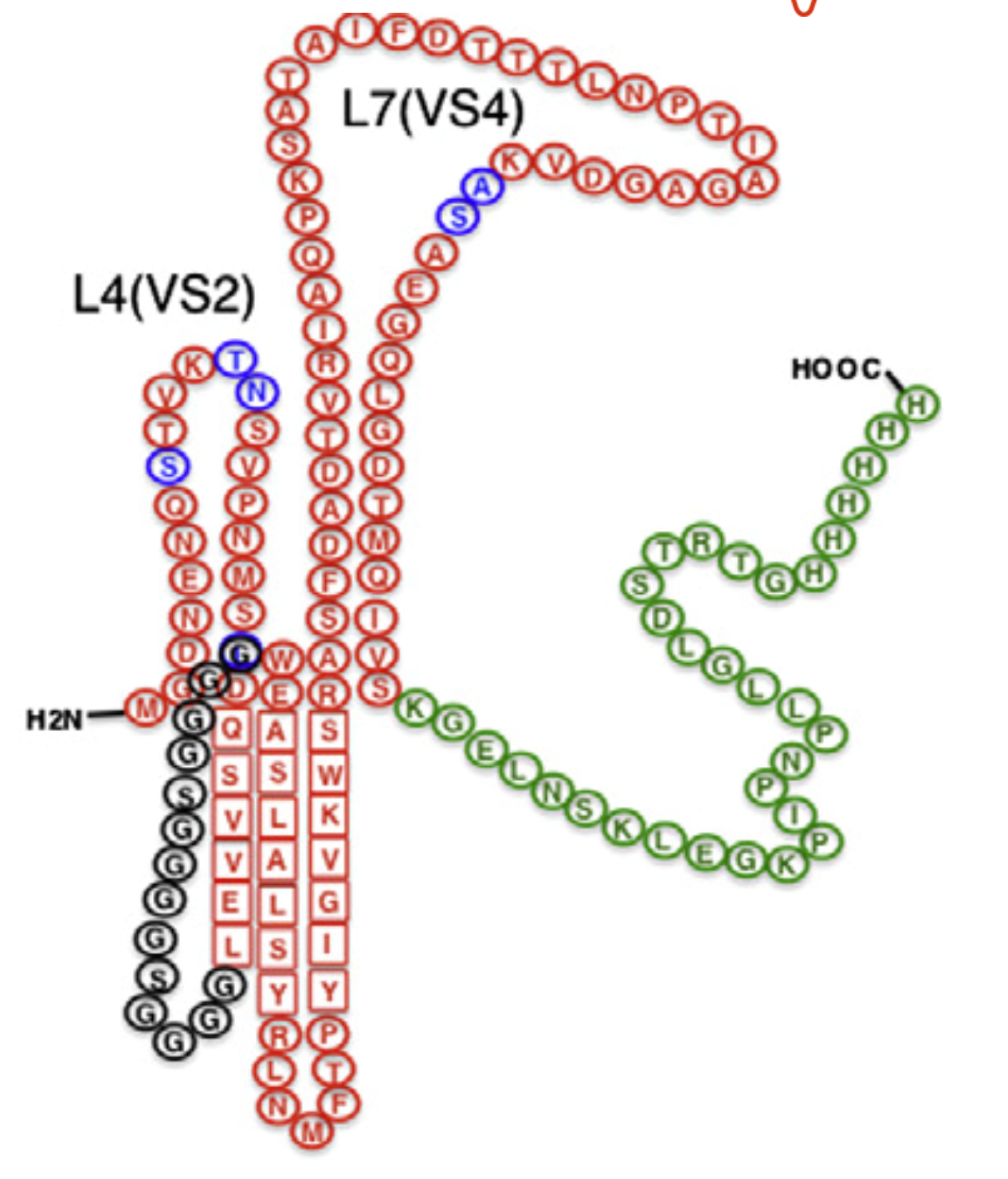
We have successfully identified sections of the MOMP (Major Outer Membrane Protein) in the bacterium Chlamydia trachomatis – sequences that can be used to trigger an immune response. The specially designed protein chains are essential for creation of the vaccine, and are patent protected.
Initial studies have proven the active protein to be very immunogenic and well tolerated even without an adjuvant.
Medical Need & Market Potential
Preventive vaccines against Chlamydia are lacking on the market today. The WHO estimates over 130 million new cases each year and a widespread implementation of vaccination in adolescents has been proposed, similar to the existing HPV vaccination programmes.
Vaccines against chlamydia are estimated to have blockbuster potential where the global sales potential for a successful vaccine against chlamydia to be comparable with the sales of HPV vaccines, where Gardasil and Cervarix dominate. Together, these two vaccines sold for more than $4.2 billion in 2020 and are now estimated to reach $6.9 billion in 2026, according to Globe Life Sciences Ltd.
At present, however, only one preventive vaccine candidate against chlamydia is in clinical development. According to the board of Eurocine Vaccines, this provides the Company with a real opportunity to establish the Company’s Chlamydia vaccine candidate as a solution to the problem.
A successful vaccine against chlamydia is also considered to contribute to a reduced use of antibiotics – chlamydia infection is today mainly treated with antibiotics – which would decrease the pressure towards antibiotic resistance, a serious threat to global public health.
Current Phase & Next Steps
Up to now, five major pre-clinical studies have been performed in mice, resulting in positive antibody and T-cell responses.
Our current and future development involves;
– to set up an industrially scalable manufacturing process to ensure high quality supply to future studies
– to manufacture study products for the planned clinical study according to GMP
– to design a Phase I clinical trial to be started in the first half of 2023
Product Line Extension
Diagnostic Test
In line with our strategy, and to capitalise our candidates even better, we now pursue the possibility of creating a Chlamydia Diagnostic Test. Since there is a lack of a verified and efficient diagnostic tool today, it both creates an additional business opportunity but is also of vital value in our forthcoming studies.
The work was initiated during 2021 and studies are planned for autumn 2022.
Read more =>
Adjuvant Technology
– Endocine ™
Natural Compounds
Endocine™ is a safe, tolerable and effective vaccine adjuvant, based on natural lipid compounds and formulated as a liposomal dispersion. The manufacture of bulk adjuvant has been established in pilot-scale at GMP conditions.
It has been evaluated with several different vaccine antigens, both viral and bacterial and found compatible with peptides, proteins, polysaccharides, VLPs, as well as split and whole pathogens.

Safe & Tolerable
Endocine™ has shown excellent safety and tolerability in more than 400 human subjects after nasal administration in five clinical trials. The natural origin of the lipid compounds is a key to safety and regulatory approval.
The technology is also well tolerated in animal models, such as mouse, rat and ferret, as has been shown in numerous preclinical studies.
Sample of publications
Intranasally administered Endocine™ formulated 2009 pandemic influenza H1N1 vaccine induces broad specific antibody responses and confers protection in ferrets.
Maltais AK, Stittelaar KJ, Veldhuis Kroeze EJ, van Amerongen G, Dijkshoorn ML, Krestin GP, Hinkula J, Arwidsson H, Lindberg A, Osterhaus AD. Vaccine. 2014 May 30;32(26):3307-15. doi: 10.1016/j.vaccine.2014.03.061. Epub 2014 Mar 30.
Endocine™, N3OA and N3OASq; three mucosal adjuvants that enhance the immune response to nasal influenza vaccination.
Falkeborn T, Bråve A, Larsson M, Akerlind B, Schröder U, Hinkula J. PLoS One. 2013 Aug 8;8(8):e70527. doi: 10.1371/journal.pone.0070527. eCollection 2013
The Eurocine L3 adjuvants with subunit influenza antigens induce protective immunity in mice after intranasal vaccination.
Pernilla Petersson, Mona Hedenskog, Denise Alves, Mia Brytting, Ulf Schröder, Annika Linde, Åke Lundkvist. Vaccine 28 (2010) 6491-6497
Safety and immunogenicity, after nasal application of HIV-1 DNA gagp37 plasmid vaccine in young mice.
Jorma Hinkula, Marie Hagbom, Britta Wahren, Ulf Schröder. Vaccine 26 (2008) 5101-5106
Intranasal immunization of young mice with a multigene HIV-1 vaccine in combination with the N3 adjuvant induces mucosal and systemic immune responses.
Andreas Bråve, David Hallengärd, Ulf Schöder, Pontus Blomberg, Britta Wahren and Jorma Hinkula. Vaccine 26 (2008) 5075-5078
DNA–VLP prime–boost intra-nasal immunization induces cellular and humoral anti-HIV-1 systemic and mucosalimmunity with cross-clade neutralizing activity.
L. Buonaguro, C. Devito, M.L. Tornesello, U. Schröder, B. Wahren, J. Hinkula and F.M. Buonaguro Vaccine 25 (2007) 5968-5977
A novel DNA adjuvant, N3, enhances mucosal and systemic immune responses induced by HIV-1 DNA and peptide immunizations.
Jorma Hinkula, Claudia Devito, Bartek Zuber, Reinhold Benthin, Denise Ferreira, Britta Wahren, Ulf Schröder. Vaccine 24 (2006) 4494-4497
Nasal boost with adjuvanted heat killed BCG or arabinomannan-protein conjugate improves primary BCG-induced protection in C57BL/6 mice.
M. Haile, B. Hamasur, T. Jaxmar, D. Gavier-Widen, M.A. Chambers, B. Sanchez, U. Schröder, Källenius, S.B. Svenson, A. Pawlowski. Tuberculosis (2005) 85, 107-114Intranasal HIV-1-gp160-DNA/gp41 peptide prime-boost immunization regimen of mice results in longterm HIV-1 neutralizing humoral, mucosal and systemic immunity.
C. Devito, B. Zuber, U. Schröder, R. Benthin, K. Okuda, K. Broliden, B. Wahren and J. Hinkula. J.Immunology (2004) 173: 7078-7089
Immunization with heat-killed Mycobacterium bovis bacille Calmette–Guerin (BCG) in EurocineTM L3 adjuvant protects against tuberculosis.
M. Haile, U. Schröder, B. Hamasur, A. Pawlowski, T. Jaxmar, G. Källenius, S.B. Svenson. Vaccine 22 (2004) 1498-1508
Intramuscular Vaccination
Vaccines against eight different pathogens have been tested intramuscularly or subcutaneously with Endocine™ with eight different vaccine candidates.
As an example, a vaccine candidate that was tested together with Eurocine Vaccines´ adjuvant Endocine™ in mice, showed that Endocine™ enhanced the antibody level 6-10 times after intramuscular injection, compared to the same vaccine without Endocine™.
Endocine™ is offered as a safe and effective intramuscular vaccine adjuvant for both human and veterinary use.
Nasal Vaccination
Vaccines against nine different pathogens have been tested intranasally with Endocine™. Including all antigen types, a total of twelve different vaccine candidates have been tested.
Ease of administration and the generation of mucosal immunity are attractive benefits of a nasal vaccine.
Endocine™ is offered as a safe and effective intranasal vaccine adjuvant for both human and veterinary use.